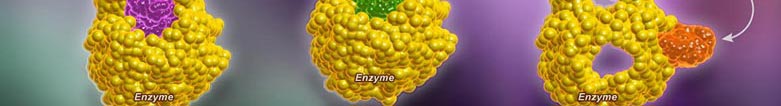
Enzyme inhibitors prevent the formation of an enzyme-substrate complex and hence prevent the formation of product. Inhibition of enzymes may be either reversible or irreversible depending on the specific effect of the inhibitor being used.
In a normal reaction, a substrate binds to an enzyme (via the active site) to form an enzyme-substrate complex. The shape and properties of the substrate and active site are complementary, resulting in enzyme-substrate specificity. When binding occurs, the active site undergoes a conformational change to optimally interact with the substrate. This conformational change destabilizes chemical bonds within the substrate, lowering the activation energy. As a consequence ofthis enzyme interaction, the substrate is converted into product at an accelerated rate.
A competitive inhibition involves a molecule, other than the substrate, binding to the enzyme’s active site. The molecule (inhibitor) is structurally and chemically similar to the substrate (hence able to bind to the active site). The competitive inhibitor blocks the active site and thus prevents substrate binding. As the inhibitor is in competition with the substrate, its effects can be reduced by increasing substrate concentration.
A non-competitive inhibition involves a molecule binding to a site other than the active site (an allosteric site). The binding of the inhibitor to the allosteric site causes a conformational change to the enzyme’s active site. As a result of this change, the active site and substrate no longer share specificity, meaning the substrate cannot bind. As the inhibitor is not in direct competition with the substrate, increasing substrate levels cannot mitigate the inhibitor’s effect.
Drugs that function as enzyme inhibitors constitute a significant portion of the orally bioavailable therapeutic agents that are in clinical use today. Most of the drug discovery and development efforts at present are focused on identifying and optimizing drug candidates that act through inhibition of specific enzyme targets. The attractiveness of enzymes as targets for drug discovery stems from the high levels of disease association (target validation) and druggability (target tractability) that typically characterize this class of proteins.
Aurora Medbiochem is proud to offer a variety of structurally diverse enzyme inhibitors for research.
A cocktail of five phosphatase inhibitors that can inhibit both acid/alkaline phosphatases and protein tyrosine phosphatases (PTPs). This cocktail is suitable for use with cell lysates as well as tissue extracts from different species
A cocktail of five phosphatase inhibitors that can inhibit both acid/alkaline phosphatases and protein tyrosine phosphatases (PTPs). This cocktail is suitable for use with cell lysates as well as tissue extracts from different species
A cocktail of three phosphatase inhibitors formulated in DMSO for the inhibition of both serine/threonine and alkaline phosphatases. Available as a 1 ml vial or as a set of five 1 ml vials. Each vial contains a 1 ml solution with the following components: 500 µM Cantharidin, 2.5 mM (-)-p-Bromotetramisole oxalate, and 1 µM Calyculin A, Discodermia calyx.
A cocktail of four phosphatase inhibitors that can inhibit both serine/threonine and protein tyrosine phosphatases (PTPs). This cocktail is suitable for use with cell lysates as well as tissue extracts from different species